What is an element? It is a substance that cannot be changed via a chemical reaction. In other words, it is what it is. Everything is made up of atoms with electrons, protons and neutrons. There are three classifications for this kind of substance: Metals, Nonmetals and Metalloids.
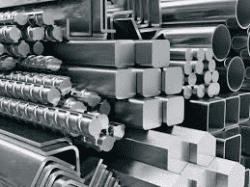
Metals are able to give and share their electrons. In fact, they have so many they are seen as floating in a sea of electrons. Metal is extremely useful as it can be ductile, meaning it can be drawn into a wire. It is malleable, meaning it can be pounded into a sheet. They also conduct electricity and heat. To do this we need to employ a Metal bonding adhesive.
Nonmetals take electrons. They are usually brittle and break with ease. They do not reflect light and don’t conduct electricity. This is not to say that they have no purpose. They make excellent insulators for electrical substances.
Metalloids. These substances sit in between the metals and the nonmetals. They share both characteristics which is extremely useful. For example silicon is a metalloid that has huge applications in computers as semiconductors. This allows the flow of power without blowing the system.
